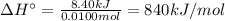Answer:
ΔH° = 840 kJ/mol
Step-by-step explanation:
Let's consider the following balanced reaction:
2 AgNO₃(aq) + CaCl₂(aq) ⇄ 2 AgCl(s) + Ca(NO₃)₂
Then, we need to know the moles of both reactants:
AgNO₃: n = 0.200 mol/L × 0.0500 L = 0.0100 mol
CaCl₂: n = 0.100 mol/L × 0.0500 L = 0.00500 mol
According to the balanced equation we need 2 moles of AgNO₃ per each mole of CaCl₂, and this coincides with the experimental data, so there is no limiting reactant. Let's use AgNO₃ to find out how many moles of AgCl are produced.
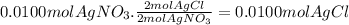
Now, we can calculate the total amount of heat released using the following expression:
Q = c × m × ΔT
where,
c is the heat capacity of the solution
m is the mass of the solution
ΔT is the change in temperature (26.0 °C - 25.0°C = 1.00 °C)
Since the volume is 100.0 mL (50.0 mL + 50.0 mL) and the density is 1.05 g/mL, we can calculate the mass of the solution like:
m = 1.05 g/mL × 100.0 mL = 105 g
Then,
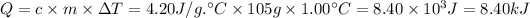
Finally,