Answer: The concentration of original sulfuric acid solution is 1.62 M
Step-by-step explanation:
Let the original concentration of sulfuric acid be 'x' M
To calculate the molarity of the diluted solution, we use the equation:

where,
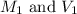 are the molarity and volume of the concentrated sulfuric acid solution
are the molarity and volume of the concentrated sulfuric acid solution
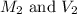 are the molarity and volume of diluted sulfuric acid solution
are the molarity and volume of diluted sulfuric acid solution
We are given:
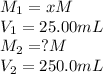
Putting values in above equation, we get:
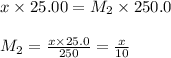
Now, to calculate the concentration of acid, we use the equation given by neutralization reaction:
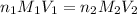
where,
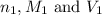 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is

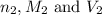 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.
We are given:
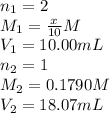
Putting values in above equation, we get:
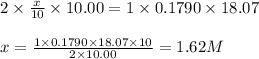
Hence, the concentration of original sulfuric acid solution is 1.62 M