The heat of reaction for combustion per gram of the fuel is approximately 23023 J/g.
How to find heat of reaction?
Calculate the total heat absorbed by the water and calorimeter:
Temperature change (ΔT) = 23.55°C - 20.00°C
= 3.55°C
Heat capacity of water (
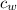 ) = 4.184 J/g°C
) = 4.184 J/g°C
Heat absorbed by water (
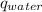 ) =
) =
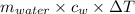
Mass of water (
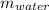 ) = 2.550 L × 1000 g/L
) = 2.550 L × 1000 g/L
= 2550 g
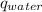 = 2550 g × 4.184 J/g°C × 3.55°C
= 2550 g × 4.184 J/g°C × 3.55°C
≈ 37145 J
Heat absorbed by calorimeter (
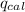 ) =
) =
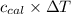
Heat capacity of calorimeter (
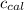 ) = 403 J/K
) = 403 J/K
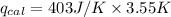
≈ 1420 J
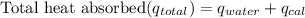
≈ 37145 J + 1420 J
≈ 38565 J
Calculate the heat of reaction per gram of fuel:
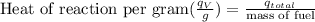
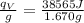
≈ 23023 J/g
Therefore, the heat of reaction for combustion per gram of the fuel is approximately 23023 J/g.