Answer:
A)
Solution:
Salt is formed when an acid reacts with a base. We call this reaction as a Neutralization reaction.
For example
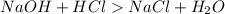
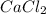 is formed from a strong base
is formed from a strong base
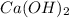 and strong acid HCl as shown in the Equation
and strong acid HCl as shown in the Equation
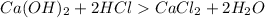
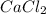 is a salt which produces ions
is a salt which produces ions
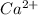 and
and
 ions into water
ions into water
So the Answer is option D
Neither
 or
or
 ions into water.
ions into water.
B)
Solution:
Arrhenius Theory: An acid is a substance which produces one or more hydrogen ions, (H+) in aqueous solution.
Examples:
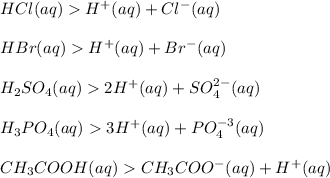
A Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples
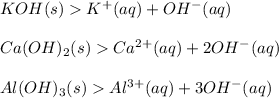
Please note: (aq) stands for aqueous which means in the presence of water that is, water acts as a solvent.
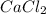 is formed from a strong base
is formed from a strong base
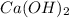 and strong acid HCl as shown in the Equation
and strong acid HCl as shown in the Equation
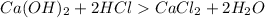
So when placed in water
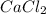 as it is formed from a strong base it releases OH- ions and as it is formed from a strong acid it releases H+ ions .
as it is formed from a strong base it releases OH- ions and as it is formed from a strong acid it releases H+ ions .
So, option C is the Answer
Equal amounts of OH- and H+ ions into water