Answer:
-6.326 KJ/K
Step-by-step explanation:
A) the entropy change is defined as:
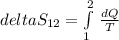
In an isobaric process heat (Q) is defined as:
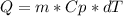
Replacing in the equation for entropy
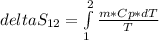
m is the mass and Cp is the specific heat of R134a. We can considerer these values as constants so the expression for entropy would be:
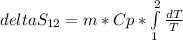
Solving the integral we get the expression to estimate the entropy change in the system
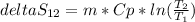
The mass is 5.25 Kg and Cp for R134a vapor can be consulted in tables, this value is
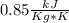
We can get the temperature at the beginning knowing that is saturated vapor at 500 KPa. Consulting the thermodynamic tables, we get that temperature of saturation at this pressure is: 288.86 K
The temperature in the final state we can get it from the heat expression, since we know how much heat was lost in the process (-976.71 kJ). By convention when heat is released by the system a negative sign is used to express it.
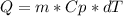
With
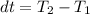 clearing for T2 we get:
clearing for T2 we get:

Now we can estimate the entropy change in the system
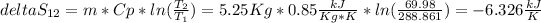
The entropy change in the system is negative because we are going from a state with a lot of disorder (high temperature) to one more organize (less temperature. This was done increasing the entropy of the surroundings.
b) see picture.