Answer:
The mass of the air is 0.0243 kg.
Step-by-step explanation:
Step1
Given:
Stroke of the cylinder is 320 mm.
Bore of the cylinder is 280 mm.
Pressure of the air is 101.3 kpa.
Temperature of the air is 13°C.
Step2
Calculation:
Stroke volume of the cylinder is calculated as follows:
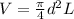
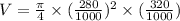
V = 0.0197 m³.
Step3
Assume air an ideal gas with gas constant 287 j/kgK. Then apply ideal gas equation for mass of the air as follows:
PV=mRT
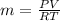
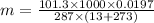
m= 0.0243 kg.
Thus, the mass of the air is 0.0243 kg.