Answer: 446000 Joules
Step-by-step explanation:
Latent heat of vaporization is the amount of heat required to convert liquid to gas at atmospheric pressure.
Latent heat of vaporization = 2230 J/g
Volume of water = 200 ml
Density of water = 1 g/ml
Mass of water = ?
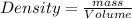
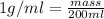
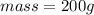
1 gram of water requires energy to evaporate = 2230 Joules
200 gram of water requires energy to evaporate =
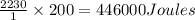
Thus 446000 Joules of energy is required to evaporate 200 mL of sweat.