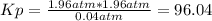Answer:
96.04
Step-by-step explanation:
Kp is defined for any reaction as:
aA(g) +bB(g) ⇌ cC(g) +dD(g)
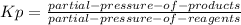
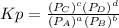
For the reaction:
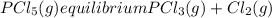
Kp is:
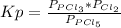
P in the equation is the partial pressure for each specie in the equilibrium.
We know the total pressure for the system at the beginning is 2 atm. We can say that at the beginning we have just
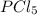 so the total pressure for the system is also the partial pressure for
so the total pressure for the system is also the partial pressure for
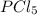
In the equilibrium we have the three species
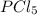 ,
,
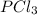 and
and
 so now we need to estimate the partial pressure for each specie.
so now we need to estimate the partial pressure for each specie.
We know that the total pressure for the system in the equilibrium is 3.96 atm. The total pressure is equal to the sum of the partial pressure of each component in the mix as:
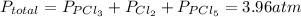
Until we reach the equilibrium
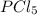 it’s been used to produce
it’s been used to produce
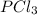 and
and
 . We can call (x) the among of
. We can call (x) the among of
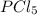 that reacted until the system reach the equilibrium so
that reacted until the system reach the equilibrium so
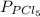 in the equilibrium will be
in the equilibrium will be
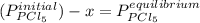
When reviewing the molar ratio in the reaction we see that it is 1: 1 so the amount of
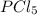 that reacted will be equal to the amount of
that reacted will be equal to the amount of
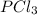 and
and
 produced.
produced.
PCl5 Cl2 PCl3
Initial 2 atm 0 atm 0 atm
Equilibrium (2 atm - x) X atm X atm
Using the equation for total pressure:
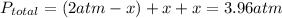
solving we get that x = 1.96 atm.
This is the partial pressure for
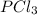 and
and
 in the equilibrium
in the equilibrium
For each component we have:
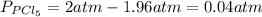
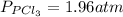
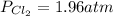
So