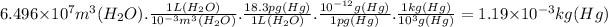Answer:
(a) The volume of water in the quarry is 6.496 x 10⁷ m³.
(b) The total mass of mercury is 1.19 x 10⁻³ kg.
Step-by-step explanation:
(a) The volume of water can be calculated like:
Volume = depth x surface
In order to have coherence between units we will use proportions to transform km² to m².
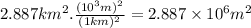
Then,
Volume = 22.50 m x 2.887 x 10⁶ m² = 6.496 x 10⁷ m³
(b) We can find out the total mass of mercury using proportions. We will need these relations:
1 L = 10⁻³ m³
1 pg = 10⁻¹² g
1 kg = 10³ g