Answer: The mass of PVB required to produce 1000 grams of tape is 213.4 grams
Step-by-step explanation:
To calculate the mass of aluminium oxide, we use the equation:
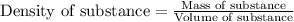 .......(1)
.......(1)
- For
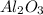
We are given:
50% (v/v) of
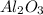
This means that 50 mL of aluminium oxide is present in 100 mL of tape
Calculating the mass of aluminium oxide by using equation 1:
Density of aluminium oxide =
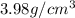
Volume of aluminium oxide =
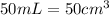 (Conversion factor:
(Conversion factor:
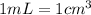 )
)
Putting values in equation 1, we get:
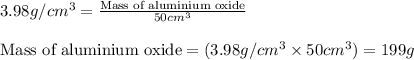
Mass of aluminium oxide = 199 g
We are given:
50% (v/v) of PVB
This means that 50 mL of PVB is present in 100 mL of tape
Calculating the mass of PVB by using equation 1:
Density of PVB =
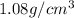
Volume of PVB =
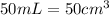
Putting values in equation 1, we get:
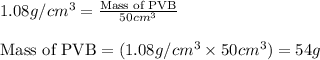
Mass of PVB = 54 g
Mass of tape = Mass of aluminium oxide + mass of PVB
Mass of tape = [199 + 54] g = 253 g
To calculate the mass of PVB required to produce 1000 g of tape, we use unitary method:
When 253 grams of tape is made, the mass of PVB required is 54 g
So, when 1000 grams of tape is made, the mass of PVB required will be =
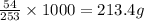
Hence, the mass of PVB required to produce 1000 grams of tape is 213.4 grams