Answer:
a)
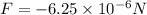 , attractive.
, attractive.
b)
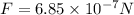 , repulsive.
, repulsive.
Step-by-step explanation:
We use Coulomb's Law to calculate the electrostatic force between 2 charges
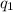 and
and
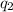 separated a distance r:
separated a distance r:
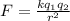
Where
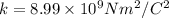 is Coulomb's constant.
is Coulomb's constant.
a) At the beginning we then have:
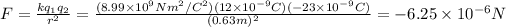
Since their signs are different it will be attractive.
b) The total charge must be conserved, which is:
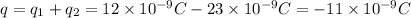
So now each charge will have a charge half this value
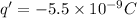
And the force will be:
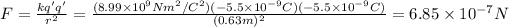
Since their signs are the same it will be repulsive.