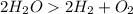Answer:
A chemical change has occurred.
Step-by-step explanation:
A chemical change is accompanied by a change in the chemical composition of the substance.
A color change, formation of a precipitate, evolution of a gas , temperature change are the indications of a chemical change.
For example
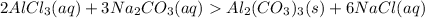
Here we see the formation of a precipitate or an insoluble solid
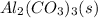
A chemical reaction is accompanied by a change in temperature.
Reaction in which heat is evolved is an exothermic reaction and reaction in which heat is absorbed is called as endothermic reaction.
Combustion reaction is an example in which heat and light is given off and it is exothermic.
Some reactions we see gas gets evolved. For example electrolysis of water produces Hydrogen and oxygen gas.