Answer:
For A: The mass of oxygen per gram of sulfur in sulfur dioxide is 0.997
For B: The mass of oxygen per gram of sulfur in sulfur trioxide is 1.5
Step-by-step explanation:
We are given:
Mass of oxygen in sulfur dioxide = 3.49 grams
Mass of sulfur in sulfur dioxide = 3.50 grams
So,
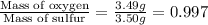
Hence, the mass of oxygen per gram of sulfur in sulfur dioxide is 0.997
We are given:
Mass of oxygen in sulfur trioxide = 9.00 grams
Mass of sulfur in sulfur trioxide = 6.00 grams
So,
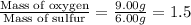
Hence, the mass of oxygen per gram of sulfur in sulfur trioxide is 1.5