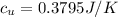Answer:
Step-by-step explanation:
The heat released by the unknown mineral (
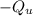 , where we will use the minus sign to indicate release of heat) is the heat absorbed by the calorimeter with water
, where we will use the minus sign to indicate release of heat) is the heat absorbed by the calorimeter with water
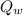 , so we can write:
, so we can write:
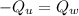
The formula that relates heat with the mass of a material, its specific heat capacity and the temperature difference it suffers because of that heat is:
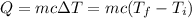
So we substitute this into the previous equation to get:
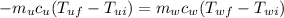
Since at the end the final temperatures will be the same, namely
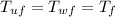
We can write
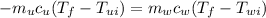
Since what we want is the specific heat capacity of the unknown mineral
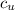 , we can write:
, we can write:
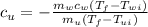
In this particular equation it is not necessary to transform g to Kg (since the units of masses are cancelling out) or Celsius to Kelvin (since temperature is only appearing as a difference), but if one is not sure, the units should be transformed to S.I. since Joules is in S.I.
Finally then we have:
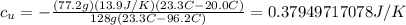
Since it asks for 4 decimal places, this must be written as: