Answer:
The atmospheric pressure is 0.843 bar.
Step-by-step explanation:
First, we calculate how much thermal energy (heat) was transferred to the water:
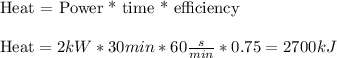
Knowing that all heat (Q) was used in making 1.19 kg of water boil (liquid to gas), we can find what is the latent heat (
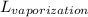 ) of this change of state (vaporization):
) of this change of state (vaporization):
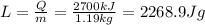
Looking up this value in a Water Heat of Vaporization Calculator, we find that it corresponds to a temperature of 94.9°C.
Knowing the temperature at which the water boils, we have to find the vapor pressure (the same as the latent heat according to temperature, it is a value which can be found in a table) at that temperature, which would be the atmospheric pressure of the location.
The vapor pressure of water at 94.9°C is 0.843 bar, i.e. the atmospheric pressure is 0.843 bar.