Answer:
NaOH is the Answer.
Step-by-step explanation:
By criss crossing the charge numbers, we find the chemical formula of a compound
For example Aluminium chloride
Al has 3+ charge and Cl has 1- charge
3 becomes the subscript of Cl and 1 becomes the subscript of Al
So the chemical formula will be
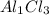
Sodium Hydroxide is NaOH as Na the charge is
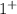 and as OH, the charge is
and as OH, the charge is

Sodium phosphate is
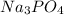 as sodium has
as sodium has
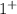 charge and phosphate has
charge and phosphate has
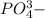 charge.
charge.
Sodium sulfate is
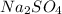 as sodium has
as sodium has
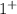 charge and sulfate has
charge and sulfate has
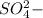 charge.
charge.
Sodium chloride will be NaCl as sodium has
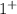 charge and chloride has
charge and chloride has
 charge.
charge.
Calcium phosphate will be
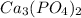 because Calcium possess
because Calcium possess
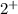 charge and phosphate has
charge and phosphate has
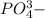 charge.
charge.