Step-by-step explanation:
It is given that 185 ml of buffer solution is 0.40 M
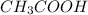 and
and
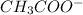 .
.
Now, according to the Handerson equation,
pH =
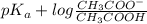
It is known that
 value of acetic acid is 4.76.
value of acetic acid is 4.76.
pH =
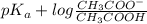
=
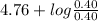
= 4.76
If pH of a buffer changes by 1 unit then it means the buffering capacity is lost.
Hence, when HCl is being added it reacts with
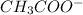 and gives
and gives
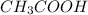 . So, with increase in
. So, with increase in
![[CH_(3)COOH]](https://img.qammunity.org/2020/formulas/chemistry/college/dhwhv8n9mznk3o80acun1w97kcl7l49702.png) the log term gives a negative value. This means that new pH will be less than 4.76.
the log term gives a negative value. This means that new pH will be less than 4.76.
Therefore, calculate the concentration when pH = 3.6.
3.76 = 4.76 +
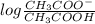
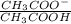 = 0.1 ....... (1)
= 0.1 ....... (1)
Now, we assume that the moles of acid added or change in moles is x. Therefore, moles of acetic acid and conjugate base present are as follows.
No. of moles = Molarity × Volume
= 0.40 × 185 ml
= 74 mmol
Now, we put this value into equation (1) as follows.
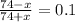
x = 60.5
This means that moles of acid added is 60.5 mmol.
As it is given that molarity is 0.180 M. Therefore, calculate the volume of acid as follows.
Volume of acid =
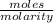
=
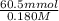
= 336.1 ml
Thus, we can conclude that the maximum volume of 0.180 M HCl that can be added to the buffer before its buffering capacity is lost is 336.1 ml.