Answer: Option (A) is the correct answer.
Step-by-step explanation:
Since, thermal energy is being added to the chemical reaction which will lead to an increase in the motion of molecules.
Hence, kinetic energy of the molecules will increase. As a result, number of collisions within the molecules will increase leading to an increase in the rate of reaction.
Also, it is known that
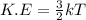
Therefore, we can conclude that the statement increase in energy will cause the reactance particles to move faster which will increase their temperature and lead to a faster reaction rate correctly describes how the addition of thermal energy will affect the reaction rate.