Answer
speed of the molecules
s₁ = v t
when velocity is doubled
s₂ = (2 v)t
= 2 s₁
they will hit the wall of container two times as often.
the momentum of molecule
p₁ = mvr
p₂ = m(2v)r = 2(mvr)
= 2 p₁
the momentum change is two times as great.
force is change in momentum
Δp = F(Δt)
mv-(-mv) = 2 mv
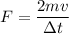
F α v
therefore average force impart to the wall on each collision is two times
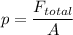
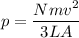
p α v²
here the velocity is doubled it means pressure becomes four times.