Answer:
We need a tank of volume 49866.6168 gallon
Step-by-step explanation:
We have given mass of chlorine m = 200 lbm
Density of chlorine
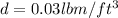
We know that mass = density × volume
So volume
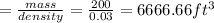
We know that 1
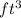 = 7.480 gallon
= 7.480 gallon
So 6666.66
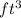 = 6666.66×7.480 = 49866.6168 gallon
= 6666.66×7.480 = 49866.6168 gallon
So we need a tank of volume 49866.6168 gallon