Answer:
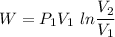
Step-by-step explanation:
Lets take
At initial position
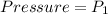
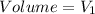
At final position
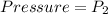
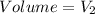
SI unit
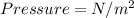
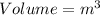
English units
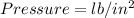
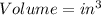
For expansion process:
As we know that work done given as
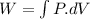
We know that for isothermal process
P.V = C
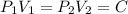
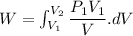
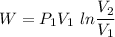
Expression for expansion and for compression will be same.