Answer:
P = 1.61 atm
Step-by-step explanation:
We need to use the Van der Waals gas formula to solve this, as it is hinted that the gas in question must be treated as a Van der Waals gas. This formula is:
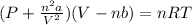
For Nitrogen (
 ), we can find from tables that the value of those constants are a=1.35 atm
), we can find from tables that the value of those constants are a=1.35 atm
 and b=0.0387 L/mol. In case they are in other units is necessary to convert them since we need to stick with the same system of units always.
and b=0.0387 L/mol. In case they are in other units is necessary to convert them since we need to stick with the same system of units always.
We can then do (dividing both sides by V-nb):
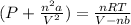
And finally have the expression for the pressure:
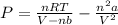
We then put the values we already have on this formula:
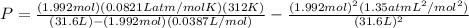
Which after careful calculation, and noticing again that we have used the same system of units for everything so they canel out properly, gives us the result P = 1.61 atm.