Answer : The value of
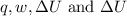 is 505 J, -599 J, -94 J and -693 J respectively.
is 505 J, -599 J, -94 J and -693 J respectively.
Explanation : Given,
Mass of steam = 7.23 g
Initial temperature =
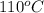
Final temperature =
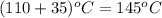
Initial volume = 2 L
Final volume = 8 L
External pressure = 0.985 bar
Heat capacity of steam = 1.996 J/g.K
First law of thermodynamic : It states that the energy can not be created or destroyed, it can only change or transfer from one state to another state.
As per first law of thermodynamic,
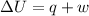
First we have to calculate the heat absorbed by the system.
Formula used :
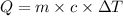
or,
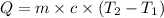
where,
Q = heat absorbed by the system = ?
m = mass of steam = 7.23 g
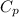 = heat capacity of steam =
= heat capacity of steam =
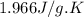
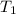 = initial temperature =
= initial temperature =
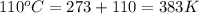
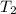 = final temperature =
= final temperature =
Now put all the given value in the above formula, we get:
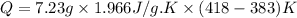
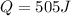
Now we have to calculate the work done.
Formula used :
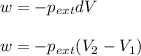
where,
w = work done = ?
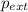 = external pressure = 0.985 bar = 0.985 atm (1 bar = 1 atm)
= external pressure = 0.985 bar = 0.985 atm (1 bar = 1 atm)
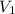 = initial volume of gas = 2.00 L
= initial volume of gas = 2.00 L
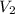 = final volume of gas = 8.00 L
= final volume of gas = 8.00 L
Now put all the given values in the above formula, we get :
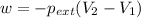
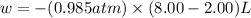
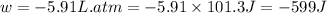
conversion used : (1 L.atm = 101.3 J)
Now we have to calculate the change in internal energy of the system.
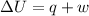
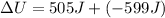
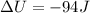
Now we have to calculate the change in enthalpy of the system.
Formula used :
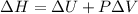

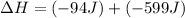
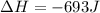
Therefore, the value of
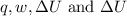 is 505 J, -599 J, -94 J and -693 J respectively.
is 505 J, -599 J, -94 J and -693 J respectively.