Step-by-step explanation:
The given value of K = 4.6
As before extraction, 40 mg of caffeine in 2 ml of water.
Hence, after the first extraction,
4.6 =
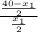
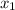 = 7.2 mg
= 7.2 mg
Therefore, caffeine extracted from dichloromethane is as follows.
40 mg - 7.2 mg = 32.8 mg
Now, after the second extraction
4.6 =
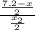
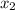 = 1.29 mg
= 1.29 mg
So, (7.2 - 1.29) mg = 5.91 mg
This means that 5.91 mg of caffeine is extracted from dichloromethane.
After the third extraction,
4.6 =

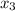 = 0.229 mg
= 0.229 mg
Hence, caffeine extracted from dichloromethane is as follows.
(1.29 - 0.229) mg
= 1.061 mg
Therefore, we can calculate the total amount of caffeine extracted as follows.
Total caffeine extracted = 40 - caffeine remains in dichloromethane after three extraction
= 40 - 0.229
= 39.771 mg
Thus, we can conclude that the total extracted caffeine is 39.771 mg.