Answer: 2.4 M
Step-by-step explanation:
According to the neutralization law,
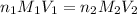
where,
 = molarity of
= molarity of
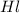 solution = 3.5 M
solution = 3.5 M
 = volume of
= volume of
 solution = 34.3 ml
solution = 34.3 ml
 = molarity of
= molarity of
 solution = ?
solution = ?
 = volume of
= volume of
 solution = 50 ml
solution = 50 ml
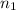 = valency of
= valency of
 = 1
= 1
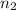 = valency of
= valency of
 = 1
= 1
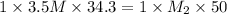
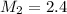
Therefore, the concentration of NaOH solution is 2.4 M.