Step-by-step explanation:
The given data is as follows.
Initial temperature of the system (
 ) =
) =
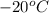
So, at state 1 quality of the system is (
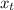 ) = 0.5036
) = 0.5036
Pressure at state 2, (
 ) = 6 bar
) = 6 bar
As, it is a rigid tank hence, the specific volumes at state 1 and state 2 are equal.
So,
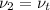
Now, from the saturated refrigerant-22 table taking specific volume at
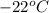 is as follows.
is as follows.
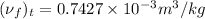
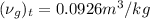
Thus,
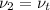
=
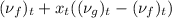
= 0.0007427 + 0.5036 (0.0926 - 0.0007427)
=
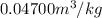
Hence,
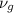 =
=
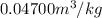
Super heated refrigerant-22 tables take pressure at 6 bar. Interpolation method used to find the temperature is as follows.
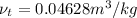 and
and
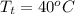
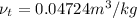 and
and
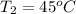
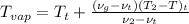
=
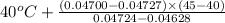
= 40 + 3.75
=
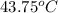
Saturated vapor refrigerant-22 takes specific volume at
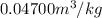 .
.
Therefore, interpolation method used to find the temperature will be as follows.
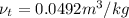 and
and
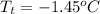
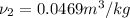 and
and
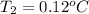
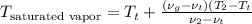
=
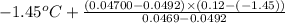
= -1.45 + 1.50
=
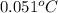
Thus, we can conclude that at
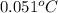 tank contains only saturated vapor.
tank contains only saturated vapor.