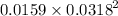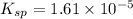Answer:
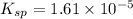
Step-by-step explanation:
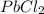 will form its respective ions in the solution as:
will form its respective ions in the solution as:
Consider the ICE take for
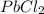 as:
as:
PbCl₂ ⇄ Pb²⁺ + 2Cl⁻
At t =equilibrium x 2x
The expression for dissociation constant of
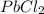 is:
is:
Solubility product =
![[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/physics/high-school/vpy6ji1rpm7ndj3km9a0jsvy4yyux46w7k.png)
Given that:
![[Pb^(2+)]=0.0159\ M](https://img.qammunity.org/2020/formulas/physics/high-school/y3g4q683jgatz6crkbat1g0o2ggfc5ybmm.png)
![[Cl^-]=0.0318\ M](https://img.qammunity.org/2020/formulas/physics/high-school/hi4uhpuwaasxws4pspp852vxiywdzhuide.png)
So,
Solubility product =
![[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/physics/high-school/vpy6ji1rpm7ndj3km9a0jsvy4yyux46w7k.png) =
=