Answer:
43%
Step-by-step explanation:
As the problem says that the antimony has only two isotopes, lets call each isotope the following:
x=abundance of isotope 121Sb
1-x=abundance of isotope 123Sb
And
Atomic weight of antimony = (isotopic mass of 121Sb*x)+(isotopic mass of 123Sb*(1-x))
Replacing values we have:
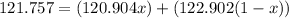
Solving for x:
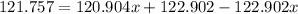
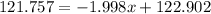
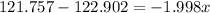
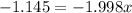
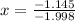
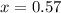
it means that the abundance of the isotope 121Sb is 57% and the abundance of isotope 123Sb is 43%