Out of the given salts,
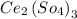 decreases in solubility as the temperature increases.
decreases in solubility as the temperature increases.
Answer: Option B
Step-by-step explanation:
Here we are talking about Cerium (III) Sulphate which is an inorganic compound, commonly known as Cerous Sulphate. Its chemical formula is
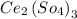 . This salt has a monoclinic crystal structure and appears off-white in colour.
. This salt has a monoclinic crystal structure and appears off-white in colour.
It’s solubility in water is 9.25 g/100ml at the temperature of 20
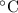 , that decreases with the increase in temperature and that’s why it’s counted amongst those few salts that has less solubility when increase temperature.
, that decreases with the increase in temperature and that’s why it’s counted amongst those few salts that has less solubility when increase temperature.