Answer:
The pressure of the gas is P=1.99 atm
Step-by-step explanation:
Given:
Amount of gas substance, n=3.0 mol
Volume of the gaseus substance V=33.6 L
To find :
Pressure of the gas =?
Solution:
According to Ideal gas Law,
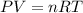
Where
p = pressure of the gas,
V = volume of the gas
n = amount of substance
R = ideal gas constant
T = temperature of the gas
Re-arranging the formula we get,
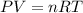
Substituting the values ,
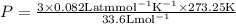
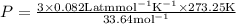
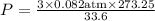
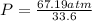
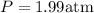
Result :
The pressure of the gas is P=1.99 atm.