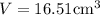Answer:
The volume of a gold piece is
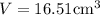
Step-by-step explanation:
Given:
Density of pure gold,
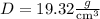
Mass of the gold piece,
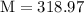
To Find:
The volume for a piece of gold whose mass is
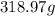
Solution:
The formula for density is:
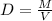
where,
D is density,
M is mass,
V is volume.
The above formula can be rewriiten as
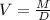
Substituting the values ,we get
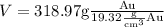
Taking multiplicative inverse of density , we have
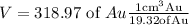
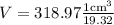
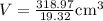
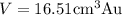
Result:
Thus the volume of gold piece weighing 318.97 g is