Answer:
The work done or the heat transfer for air and hydrogen are 91.71 kj/kg and 1317.89 kj/kg respectively.
Step-by-step explanation:
Given:
Initial pressure is 7.6 bar.
Initial temperature is 77 °C.
Final pressure is 3.05 bar.
Calculation:
(a)
Take air as an ideal gas with gas constant 0.287 kj/kgK.
Step1
For isothermal process work done and heat transfer is the same because of no change in internal energy.
Work done is calculated as follows:
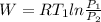
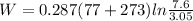
W = 91.71 kj/kg.
Thus, the work done or the heat transfer for isothermal process is 91.71 kj/kg.
Step2
For isothermal process work done and heat transfer is the same because of no change in internal energy. Take Hydrogen as an ideal gas with gas constant 4.1242 kj/kgK.
Work done is calculated as follows:
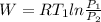
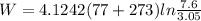
W = 1317.89 kj/kg.
Thus, the work done or the heat transfer for isothermal process is 1317.89 kj/kg.