Answer:
The equation it's very simple and corresponds to the ideal gas model, which is this one:
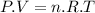
Step-by-step explanation:
Gases tend to behave following the mathematic relationship due to the ideal gas formula shown above; where P is the pressure applied to a gas inside a recipient (for example), V is the volume of the recipient where the gas exists (that is the same volume of the gas since any gas tends to fill all the volume of a limited space of the recipient), n is the number of moles and indicates the amount of gas (molecules of gas) inside the recipient and T is the temperature of that particular gas. R is just a constant called the gas constant (
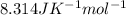 ). An ideal gas doesn't lose its internal energy over time, so the collision between the particles of the gas are considered perfect elastic collisions; which means that the system gas-recipient is a closed physical system that won't release energy to the surroundings,
). An ideal gas doesn't lose its internal energy over time, so the collision between the particles of the gas are considered perfect elastic collisions; which means that the system gas-recipient is a closed physical system that won't release energy to the surroundings,
Getting back to the actual question after the background: as n and R are constant, the pressure and temperature are directly correlated to each other, consider we assume V can't change; when the T drops, so does the pressure (think of it as the gas contracts itself because is losing excitation from the source of temperature). In other hand, if T increases, the gas will tend to expand itself so it will also increase the pressure (the gas is now colliding a lot inside the recipient because is gaining energy from the source of temperature)