Answer:
Δu= 29.82 Btu/lb
Step-by-step explanation:
Given that gas temperature changes from 50 °C to 150 °C.
K=1.4
We know that
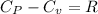
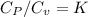
K=1.4 it means that it is air.
We know that for air
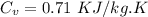
We know that internal energy for gas given as
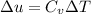 KJ/kg
KJ/kg
Δu= 0.71 x (150-50) KJ/kg
Δu=71 KJ/kg
We know that
1 KJ/kg=0.42 Btu/lb
So 71 KJ = 29.82 Btu/lb
Δu= 29.82 Btu/lb