Answer:
1,39 L
Step-by-step explanation:
Charle's Law states that the volume of a fixed amount of gas maintained at constant pressure is directly proportional to the absolute temperature of the gas, for a constant amount of gas we can write:
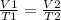
As the pressure of the balloon doesn't change, we can use Charle's Law to solve the problem. Firs we change the given temperatures to absolute temperature units ( °K), using the following relations:
°K=273+°C
°K=5/9(°F-32)+273
Therefore:
V1=1.5 L, T1=273+25=298°K
V2=?, T2=5/9(36-32)+273=275,2°K
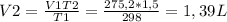
The new volume of the balloon is 1,39 L.