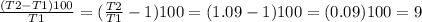Answer:
9%
Step-by-step explanation:
An ideal gas is one that has its molecules widely dispersed and does not interact with each other, studies have shown that air behaves like an ideal gas, so the state change equation for ideal gases can be applied.
P1V1T2 = P2V2T1
where 1 corresponds to state 1 = 320kPa
and 2 is state 2 = 349kPa.
Given that the volume remains constant the equation is:
P1T2=P2T1
SOLVING for T2/T1
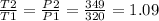
The equation to calculate the percentage increase is as follows
%ΔT=