Answer:
pressure at depth 3 m is 33.255 kPa
Step-by-step explanation:
given data
temperature = 25°C
depth = 3 m
to find out
pressure
solution
we know pressure formula that is
pressure = ρ g h ................1
and we know specific gravity of Ethylene glycol is 1.13
so
1.13 =
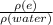
ρ (Ethylene glyco) = 1130 Kg/m³
so
pressure will be by equation 1
pressure = 1130 × 9.81 × 3
pressure = 33255.9 Pa
so pressure at depth 3 m is 33.255 kPa