Answer:
The balanced Chemical equation is
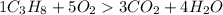
Since quantity of two reactants are given Hence we find the moles of product using both.
The quantity of product produced is the one we consider from the limiting reactant.
Limiting reactant is the reactant which runs out first.
So, least product is produced from the limiting reactant.
Mole ratio of
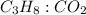 is 1: 3
is 1: 3
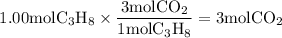
Mole ratio of
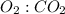 is 5: 3
is 5: 3
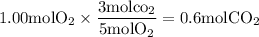
(Least produced)
So, the limiting reactant is

The amount of
 formed is 0.600mol (Answer)
formed is 0.600mol (Answer)