Step-by-step explanation:
The balanced equation for the given reaction is as follows.
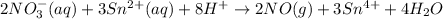
Number of moles of
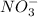 consumed will be calculated as follows.
consumed will be calculated as follows.
No. of moles =
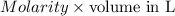
=
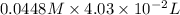
=
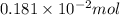
From the balanced equation, we get to know that 2 moles of
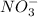 reacts with 3 moles of
reacts with 3 moles of
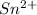 .
.
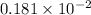 moles of
moles of
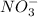 reacts with M moles of
reacts with M moles of
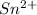 .
.
M =
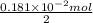
=
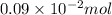
It is known that molar mass of tin is 118.71 g/mol. Hence, mass of Sn reacted will be as follows.
m =
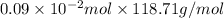
=
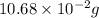
So, percent mass of tin in the original sample =
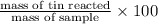
=
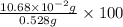
= 20.23 %
Thus, we can conclude that mass of tin is 20.23 %.