Answer:
Step-by-step explanation:
Activation energy is defined as that energy which is required by the reactants to convert it into products.
The potential energy curve for the forward and backward reaction is drawn below.
where,
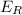 = Energy of the reactants
= Energy of the reactants
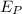 = Energy of the products
= Energy of the products
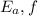 = Activation energy for the forward reaction
= Activation energy for the forward reaction
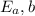 = Activation energy for the backward reaction
= Activation energy for the backward reaction
 = Amount of heat absorbed or released during the reaction
= Amount of heat absorbed or released during the reaction
 for the forward reaction is -66 kJ.
for the forward reaction is -66 kJ.
Therefore, energy of the products will be lower as compared to the energy of the products for the forward reaction.
Activation energy for the backward reaction

 = 66 kJ
= 66 kJ
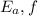 = 7 kJ
= 7 kJ
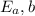 = 73 kJ
= 73 kJ