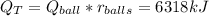Answer:
Q=6318kJ
Step-by-step explanation:
First, wirte all units in the international system:
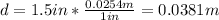
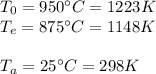
Now, check on a book to find density and specific heat of stainless steel:
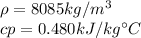
You can calculate the mass of the balls as:
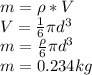
To know the heat transfer per ball:
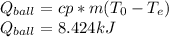
And finally to calculate the total heat transfer just multiply by the rate of balls being quenched: