Answer: The average atomic mass of an element is 35.67 amu and the unknown element will be chlorine.
Step-by-step explanation:
Mass of isotope 1 = 34.969 amu
% abundance of isotope 1 = 64.88% =
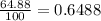
Mass of isotope 2 = 36.966 amu
% abundance of isotope 2 = (100-64.88)% =
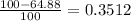
Formula used for average atomic mass of an element :
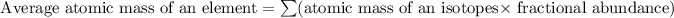
![A=\sum[34.969* 0.6488)+(36.966 * 0.3512]]](https://img.qammunity.org/2020/formulas/physics/middle-school/ge2z0tqfjwgpj0cotr10t6qht839wjru6l.png)
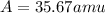
Therefore, the average atomic mass of an element is 35.67 amu and the unknown element will be chlorine.