Answer:
Step-by-step explanation:
Given parameters:
Mass of MgCl₂ = 2.52 x 10⁻⁴g
Volume of water = 2L
Unkown:
Concentration of MgCl₂ =?
Concentration of Mg²⁺ = ?
Concentration of Cl⁻ =?
Solution:
Concentration is defined as the number of moles of a solute contained in a solution.
Concentration =
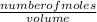
To find the number of moles"
number of moles =
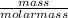
Molar mass of MgCl₂ = 24.3 + (2 x 35.5) = 95.3g/mol
number of moles =
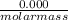 = 0.000252moles
= 0.000252moles
Concentration of MgCl₂ =
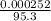 = 2.64 x 10⁻⁶moldm⁻³
= 2.64 x 10⁻⁶moldm⁻³
from the formula of the compound;
1 mole of MgCl₂ contains 1 mole of Mg²⁺
Therefore, 2.64 x 10⁻⁶moldm⁻³ of MgCl₂ will contains 2.64 x 10⁻⁶moldm⁻³ of Mg
Also;
1 mole of MgCl₂ contains 2 mole of Cl⁻
2.64 x 10⁻⁶moldm⁻³ contains 2 x 2.64 x 10⁻⁶moldm⁻³; 5.29 x 10⁻⁶moldm⁻³
Expressing in ppm;
1ppm = 1mg/L
2.64 x 10⁻⁶moldm⁻³ to mg/L for Mg²⁺
2.64 x 10⁻⁶moldm⁻³ = 2.64 x 10⁻⁶moldm⁻³ x molar mass(g/mol)
= 2.64 x 10⁻⁶moldm⁻³ x 24.3 = 6.43 x 10⁻⁵g/L
g/L to mg/L; 6.43 x 10⁻⁵g/L x 1000 = 6.43 x 10⁻²mg/L = 6.43 x 10⁻²ppm
5.29 x 10⁻⁶moldm⁻³ to mg/L for Cl⁻;
5.29 x 10⁻⁶moldm⁻³ = 5.29 x 10⁻⁶moldm⁻³ x 35.5 = 1.88 x 10⁻⁴g/L
g/L to mg/L; 1.88 x 10⁻⁴g/L x 1000 = 1.88 x 10⁻¹mg/L = 1.88 x 10⁻¹ppm