Step-by-step explanation:
Expression for vertical fluid pressure is as follows.
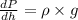 ........... (1)
........... (1)
where, p = pressure
 = density
= density
g = acceleration due to gravity
h = height
Since, g is negative then it means that an increase in height will lead to decrease in pressure.
Also, expression for density according to ideal gas law is as follows.
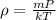 .......... (2)
.......... (2)
where, m = average mass or air molecule
P = pressure at a given point
k = Boltzmann constant
T = temperature in kelvin
Substituting values from equation (2) into equation (1) as follows.
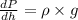
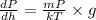
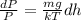
Now, on applying integration on both the sides we get the following.

or,
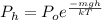
where,
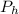 = pressure at height h
= pressure at height h
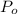 = pressure at reference point
= pressure at reference point
Thus, we can conclude that the correlation between pressure and elevation for an ideal gas is
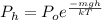 .
.