Step-by-step explanation:
The given data will be as follows.
7 kg of dimethyl terephthalate + 5 kg of Ethylene glycol = 12 kg of product
Formula for weight average number is as follows.
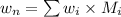
=
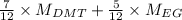
Molecular weight of DMT = 198.21 g/mol
Molecular weight of ET = 62.07 g/mol
Hence, calculate the weight average number as follows.
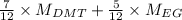
=
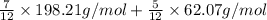
= (115.62 + 25.862) g/mol
= 141.482 g/mol
Since, efficiency is given as 50% = 0.5
0.5 =
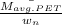
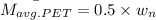
=
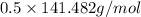
= 70.741 g/mol
As,
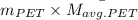 =
=
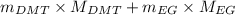
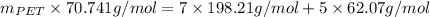
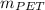 =
=
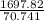
= 24 g
Thus, we can conclude that the amount of given polymer is 24 g.