Answer:
1.8321 kg
Step-by-step explanation:
The given 1 kg of coal contains 50% of the carbon atom by mass. Thus, mass of carbon in coal is
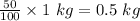
Also, 1 kg = 1000 g
So, mass of carbon = 500 g
The formula for the calculation of moles is shown below:

Molar mass of carbon = 12.0107 g/mol
Moles of methanol = 500 g / 12.0107 g/mol = 41.6295 moles
Considering the reaction:
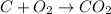
From the reaction,
1 mole of C react to form 1 mole of

So,
41.6295 moles of C react to form 41.6295 moles of

Moles of
 = 41.6295 moles
= 41.6295 moles
Molar mass of
 = 44.01 g/mol
= 44.01 g/mol
So, Mass = Moles × Molar mass = 41.6295 moles × 44.01 g/mol = 1832.1143 g
Also, 1g = 0.001 kg
So, amount of
 released = 1.8321 kg
released = 1.8321 kg