Explanation :
As we know that the Gibbs free energy is not only function of temperature and pressure but also amount of each substance in the system.

where,
 is the amount of component 1 and 2 in the system.
is the amount of component 1 and 2 in the system.
Partial molar Gibbs free energy : The partial derivative of Gibbs free energy with respect to amount of component (i) of a mixture when other variable
 are kept constant are known as partial molar Gibbs free energy of
are kept constant are known as partial molar Gibbs free energy of
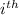 component.
component.
For a substance in a mixture, the chemical potential
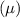 is defined as the partial molar Gibbs free energy.
is defined as the partial molar Gibbs free energy.
The expression will be:

where,
T = temperature
P = pressure
 is the amount of component 'i' and 'j' in the system.
is the amount of component 'i' and 'j' in the system.