Step-by-step explanation:
It is known that in reversible isothermal compression, relation between work and pressure is as follows.
w = -2.303 RT log
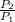
=
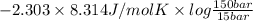
=
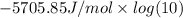
= -5705.85 J /mol
According to first law of thermodynamics, q = -w
Hence, q = -(-5705.85 J /mol)
= 5705.85 J /mol
As 1000 J = 1 kJ. Hence, convert 5705.85 J/mol into kJ/mol as follows.
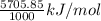
= 5.7058 kJ/mol
Thus, we can conclude that heat removal is 5.7058 kJ/mol and work required is -5705.85 J /mol.