Step-by-step explanation:
The given data is as follows.
Mass of gold = 645 lb, Volume = 0.5348 ft3
Density of water = 62.4 lbs/ft3
It is known that specific gravity is defined as density of substance divided by the density of standard fluid.
Mathematically, Specific gravity =
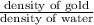
Specific gravity =
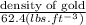
Now, calculate the density of gold then from density we will calculate specific gravity as follows
Since, Density =

Density =
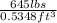
= 1206.06
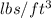
As, Specific gravity =
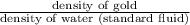
=
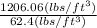
= 19.32
Therefore, the value of specific gravity is 19.32.
Specific gravity has no units as it is density divided by density. Hence, all the units get canceled out.