Step-by-step explanation:
(a) The given data is as follows.
mass = 1 kg = 1000 g (as 1 kg = 1000 g)
Molar mass of
 = 17 g/mol
= 17 g/mol
 = 2.5 bar =
= 2.5 bar =
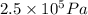 (as 1 bar =
(as 1 bar =
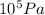 )
)
 = 5 bar =
= 5 bar =
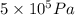
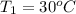 = 30 + 273 = 303 K
= 30 + 273 = 303 K
For adiabatic process,
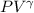 = constant = k
= constant = k
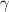 = 1.33 =
= 1.33 =
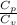
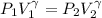
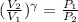
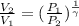
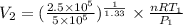 (as PV = nRT)
(as PV = nRT)
=
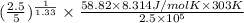
= 0.352

Also, w =
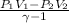
=
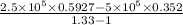
= -84318.2 J
As 1 kJ = 1000 J. So, -84318.2 J = -84.318 kJ
Hence, the work required in kJ is -84318.2 J.
(b) It is known that for adiabatic system Q = 0,
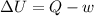
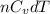 = -w
= -w
dT =
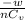
=
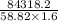
= 895.93 K
We known that dT =
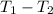
so, 895.93 = 303 K -

 = (895.93 - 303)K
= (895.93 - 303)K
= 592.93 K
= (592.93 - 273.15)^{o}C
=
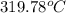
Hence, the final temperature is
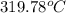 .
.